Home › Forum Online Discussion › General › Scott Kelly: NASA Twins Study Confirms Astronaut's DNA Actually Changed in Space
- This topic has 5 replies, 1 voice, and was last updated 6 years, 8 months ago by
c_howdy.
-
AuthorPosts
-
March 12, 2018 at 3:41 pm #51781
c_howdy
Participant
http://www.newsweek.com/scott-kelly-astronauts-nasa-dna-838535
By Katherine Hignett On 3/9/18 at 1:28 PM
Astronaut Scott Kelly’s DNA was altered by a year in space, results from NASA’s Twins Study have confirmed. Seven percent of his genes did not return to normal after he landed, researchers found.
Scott Kelly and his twin brother, Mark Kelly—also an astronaut—were the subjects of the study that sought to find out exactly what happens to the body after a year in space.
Scott stayed on the International Space Station from March 2015 to March 2016, while Mark remained on Earth. This was the final mission for Scott, who spent a total of 520 days in space during his career.
Scott Kelly’s one-year stint in space is “a stepping stone to a three-year mission to Mars,” NASA reported. At present, astronauts only spend six months on the International Space Station as standard. A mission to Mars, however, could take three years.
Researchers studied Scott in space psychologically and physiologically, comparing his results to those of his Earthbound brother. They looked at various proteins and evaluated the twins’ cognition as part of the overall study. Ten research teams presented their preliminary findings last year at NASA’s Human Research Program 2017 Investigators’ Workshop (IWS). The recent 2018 IWS saw these findings confirmed. Researchers also presented data from Scott’s time back on Earth.
The researchers linked space travel to oxygen deprivation stress, increased inflammation and striking nutrient shifts that affect gene expression. Some of these changes went back to normal within hours of landing on Earth. A few, however, still affected Scott six months after his return.
In 2017, researchers discovered that the endcaps of Scott Kelly’s chromosomes—his telomeres—had become longer while he was in space. Further testing confirmed this change, and revealed that most of the telomeres had shortened again within just two days of his return.
After landing, 93 percent of Scott Kelly’s genes returned to normal, the researchers found. The altered 7 percent, however, could indicate long-term changes in genes connected to the immune system, DNA repair, bone formation networks, oxygen deprivation and elevated carbon dioxide levels.
The individual studies on the twins will be combined into a summary paper, as detailed in the graphic above. This summary is set to be released later this year. The research will inform NASA’s understanding of the human body in space for “years to come,” the agency reported, as it “continues to prioritize the health and safety of astronauts on spaceflight missions.”

 March 12, 2018 at 6:11 pm #51782
March 12, 2018 at 6:11 pm #51782c_howdy
Participant…in 2017, researchers discovered that the endcaps of Scott Kelly’s chromosomes—his telomeres—had become longer while he was in space. Further testing confirmed this change, and revealed that most of the telomeres had shortened again within just two days of his return…
-https://en.wikipedia.org/wiki/Telomere#Shortening-
Telomeres shorten in part because of the end replication problem that is exhibited during DNA replication in eukaryotes only. Because DNA replication does not begin at either end of the DNA strand, but starts in the center, and considering that all known DNA polymerases read the template strand in the 3′ to 5′ direction, one finds a leading and a lagging strand on the DNA molecule being replicated…telomere shortening is associated with aging, mortality and aging-related diseases. In 2003, Richard Cawthon discovered that those with longer telomeres lead longer lives than those with short telomeres. However, it is not known whether short telomeres are just a sign of cellular age or actually contribute to the aging process themselves.
 March 12, 2018 at 7:14 pm #51783
March 12, 2018 at 7:14 pm #51783c_howdy
Participant…the research will inform NASA’s understanding of the human body in space for “years to come,” the agency reported, as it “continues to prioritize the health and safety of astronauts on spaceflight missions”…
 March 12, 2018 at 10:01 pm #51785
March 12, 2018 at 10:01 pm #51785c_howdy
ParticipantHow to build a better railway—in (almost) every cell in your body
March 12, 2018, University of Warwick
New work from the University of Warwick shows how a microscopic ‘railway’ system in our cells can optimise its structure to better suit bodies’ needs.
The work was conducted by Professor Robert Cross, director of the centre for mechanochemical cell biology at Warwick Medical School and leader of the Cross lab.
His team based at Warwick Medical School has been looking at how the microtubule ‘railway tracks’ inside cells are built. Almost every cell in our bodies contains a ‘railway’ network, a system of tiny tracks called microtubules that link important destinations inside the cell. Professor Cross’ team found the system of microtubule rails inside cells can adjust its own stability depending on whether it is being used or not..
Prof Cross said: “The microtubule tracks of the cellular railway are almost unimaginably small – just 25 nanometres across (a nanometre being a millionth of a millimetre).The railway is just as crucial to a well-run cell as a full-size railway is to a well-run country. For cells and for countries the problem is very much the same – how to run a better railway?”
“Imagine if the tracks of a real railway were able to ask themselves, ‘am I useful?’ To find out, they would check how often a railway engine passed along them.
“It turns out that the microtubule railway tracks inside cells can do exactly that – they check whether or not they are in contact with tiny railway engines (called kinesins). If they are, then they remain stably in place. If they are not, they disassemble themselves. We think this allows the sections of microtubule rail to be recycled to build new and more useful rails elsewhere in the cell.”
The paper, ‘Kinesin expands and stabilizes the GDP-microtubule lattice’ published (12 March 2018) in Nature Nanotechnology, shows that when the kinesin railway engines contact their microtubule rails, they subtly change their structure, producing a very slight lengthening that stabilises the rail.
Using a custom built microscope, the Warwick Open Source Microscope, the researchers who are also based at Warwick Systems Biology Centre and Mathematics Institute, University of Warwick, detected a 1.6% increase in the length of microtubules attached to kinesins, with a 200 times increase in their lifetime.
By revealing how microtubules are stabilised and destabilised, the team hope to throw new light on the workings of a number of human diseases (for example Alzheimer’s), which is linked to abnormalities in microtubule function. They are hopeful also that their work may ultimately lead to improved cancer therapy because the railway is so vital (for example for cell division), as its microtubule tracks are a key target for cancer drugs such as Taxol. Exactly how Taxol stabilises microtubules in cells remains poorly understood.
Professor Cross added: “Our new work shows that the kinesin railway engines stabilise microtubules in a Taxol-like way. We need to understand as much as we can about how microtubules can be stabilised and destabilised, to pave and illuminate the road to improved therapies.”
More information: Daniel R. Peet et al, Kinesin expands and stabilizes the GDP-microtubule lattice, Nature Nanotechnology (2018). DOI: 10.1038/s41565-018-0084-4
Journal reference: Nature Nanotechnology
Provided by: University of Warwick
 March 13, 2018 at 9:50 pm #51788
March 13, 2018 at 9:50 pm #51788c_howdy
Participanthttps://www.youtube.com/watch?v=WTocmrMxVDw
Mitochondria may metabolize ADP differently in aging muscle, despite exercise resistance
March 13, 2018, Cell Press
Most adults reach their peak levels of muscle mass in their late 30s or early 40s. Even for those who exercise regularly, strength and function start to decline after that point. For those who don’t exercise, the drops can be dramatic. Now, a study of twenty men published March 13 in the journal Cell Reports provides new clues about the cellular mechanisms of aging muscles, showing a key role for how mitochondria, the powerhouses of the cell, process ADP, which provides energy to cells.
ADP, or adenosine diphosphate, plays a role in how our cells release and store energy. But previous lab models that have looked at the mechanisms of aging in human cells have not included ADP. When ADP is metabolized in the mitochondria, it stimulates cellular respiration and decreases reactive oxidative species (ROS; also known as free radicals). Higher ROS levels are linked to damage in different components of the cell, a process also called oxidative stress.
In the study, the investigators developed an in vitro system employing individual muscle fibers taken from muscle biopsies. The fibers were put into a system in which mitochondrial function and respiration could be measured across a range of ADP concentrations that are relevant to those found in the human body. “The way people normally measure ROS is in a system that has ADP removed,” says senior author Graham Holloway, an associate professor at the University of Guelph in Ontario. “But biologically, we always have ADP in the system. We started to think that maybe how we get ADP into the mitochondria is important for aging.”
In the first part of the paper, the researchers compared muscle from ten healthy men in their 20s with muscle from ten healthy men in their early 70s. They found that there was an 8- to 10-fold decrease in ADP sensitivity, and therefore, when ADP was added to the system, there was a 2- to 3-fold higher rate of ROS emission in the muscle taken from the older men. ROS levels were determined by measuring emissions of hydrogen peroxide, a byproduct of activity in the cell.
The findings suggested that mitochondrial ADP sensitivity was somehow impaired in the muscles of the older men and that increased levels of ROS were contributing to sarcopenia, or the degenerative loss of muscle mass. “The magnitude of change was quite striking to us,” Holloway explains. “For humans, it’s remarkable to have such a big difference.”
In the second part, the older men undertook a program of supervised resistance training, which included leg presses and upper-body exercises. But, after 12 weeks, there were no changes in the levels of hydrogen peroxide emitted, suggesting no improvements in age-associated cellular stress.
“This doesn’t mean there’s no hope for building strength in aging muscle,” Holloway says. “I actually think that endurance training would be potentially beneficial, because we know with that kind of training you get increases in mitochondrial content.” Endurance training includes aerobic exercise like cycling and swimming. “Moving forward, we plan to look at other types of exercise, to see if it can improve the dynamic response of mitochondria to ADP,” he adds.
Other future work will use rodent models to delve into the cause-and-effect relationships of the molecular mechanisms of ADP metabolism. The investigators also plan to extend their studies to looking at different types of exercise in aging women. Early research in healthy young people has indicated that there are differences in sensitivity to ADP between men and women.
More information: Cell Reports, Holloway et al. “Age-associated impairments in mitochondrial ADP sensitivity contribute to redox stress in senescent human skeletal muscle.” http://www.cell.com/cell-reports/fulltext/S2211-1247(18)30264-X , DOI: 10.1016/j.celrep.2018.02.069
March 15, 2018 at 1:01 am #51789c_howdy
ParticipantCells stressed out? Make mitochondria longer
March 14, 2018, The Scripps Research Institute
https://phys.org/news/2018-03-cells-stressed-mitochondria-longer.html
Scientists at The Scripps Research Institute (TSRI) have discovered a new pathway in cells that promotes mitochondrial function during times of stress, a response that can guard against disease as we age.
In response to stress, rather than churn out misshapen proteins, our cells activate protective pathways that take an even more dramatic response—shutting down protein production entirely. Researchers show that along with this shutdown comes an odd change in shape of organelles called mitochondria, which are responsible for generating cellular energy. Instead of looking like tiny lima beans, mitochondria start to stretch out like noodles.
“Just a couple hours of not making proteins seems to be enough to remodel the mitochondria, and they can stay that way for hours,” says Luke Wiseman, PhD, associate professor at TSRI and senior author of the new study, published this week in the journal Cell Reports. “That seems to be a protective way to promote mitochondrial function during the early stages of stress.”
The new study offers a closer look at a stress-response pathway in cells called the Unfolded Protein Response (UPR). The UPR has several “branches” that regulate different cellular functions. The Wiseman lab focuses on how stress in a compartment of cells called the endoplasmic reticulum (ER) affects mitochondrial shape and function.
An important player in this response is a sensor/initiator of the UPR called PERK. Wiseman describes the PERK branch as a finely tuned signaling pathway. Without enough PERK signaling, the mitochondria can go haywire in times of stress and significantly challenge cellular function. But if this pathway is hyperactivated, the cell self-destructs.
As we age however, it becomes difficult for the system to maintain this balance. “When you’re older, little problems can become bigger problems because the PERK pathway isn’t as good at responding,” Wiseman says.
Previous research shows that in times of stress, PERK has an important role in regulating many aspects of mitochondrial function including preventing the mitochondrial accumulation of misshapen proteins in response to ER stress. This new study shows that shutting down protein production through activation of PERK also influences mitochondrial shape by increasing its length. Changes in mitochondrial shape are known to influence mitochondrial function, indicating that this is a mechanism to adapt mitochondrial function during ER stress.
The next question for the team was whether this shutdown and remodeling was helping or hurting cells. The mitochondria’s main role is to produce energy for the cell, so the researchers measured energy output to see how well mitochondria were functioning after cells experienced ER stress.
They found that shutting down protein production and remodeling the mitochondria did make a difference. “We were able to able to show a protective effect, where mitochondrial energy production was protected due to increased mitochondrial length” says Justine Lebeau, PhD, research associate at TSRI and co-first author of the study.
The researchers suspect that this whole system evolved to give cells a way to respond to stress very quickly, when they just don’t have time to make a batch of protective proteins.
“Blocking protein synthesis—and promoting cellular energy levels by regulating mitochondrial shape—seems to be an effective way of combatting stress over shorter time scales,” says Aparajita Madhavan, graduate student at TSRI and co-first author of the study.
Wiseman thinks defects in PERK sensitivity/activation caused by aging or mutations might hinder this protective regulation of mitochondria. He says defects in PERK signaling are implicated in many diseases that also include mitochondrial dysfunction, such as diabetes, heart disease, and neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. He hopes the new work could point to a way to target this aspect of PERK signaling to correct mitochondria defects that cause
More information: Justine Lebeau et al, The PERK Arm of the Unfolded Protein Response Regulates Mitochondrial Morphology during Acute Endoplasmic Reticulum Stress, Cell Reports (2018). DOI: 10.1016/j.celrep.2018.02.055
Journal reference: Cell Reports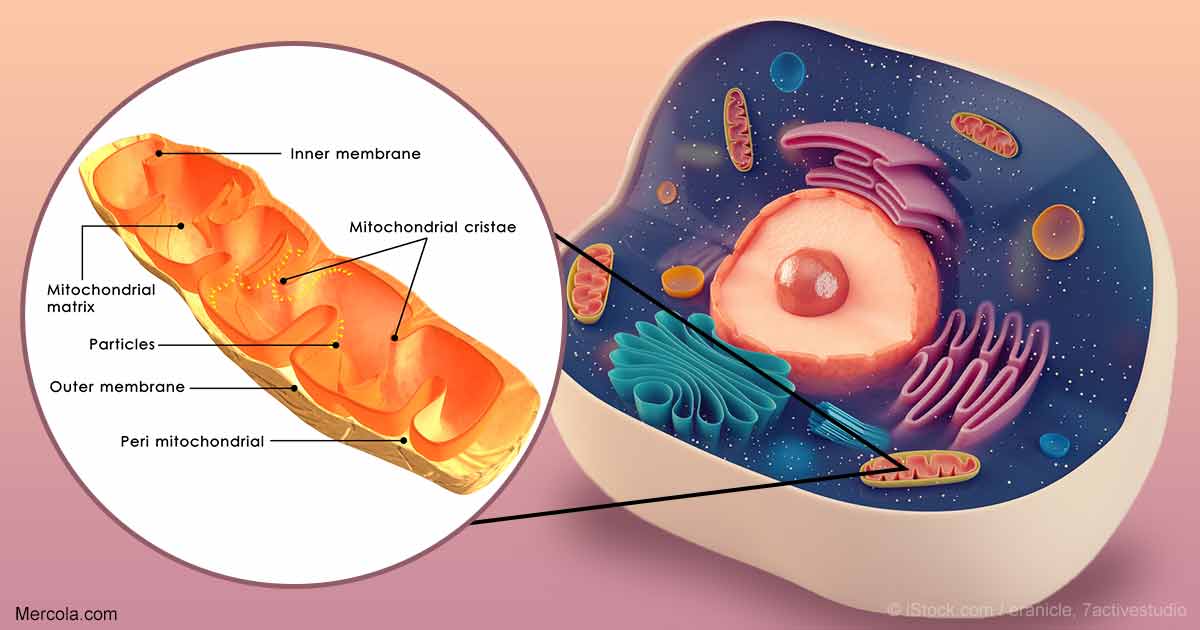
-
AuthorPosts
- You must be logged in to reply to this topic.